Big Reuse fights climate change through community-based zero waste initiatives.


Save Queensbridge Composting Site
ParksNYC has plans to repurpose Queensbridge composting site into a parking lot, disregarding the positive environmental impact and community support the our site has garnered in over a decade. Sign the petition to stand up for a greener, more sustainable city.

Our voices are making an impact! Support continues growing to restore funding to community composting and outreach.
NYC cut ALL funding for community composting and outreach. Due to these cuts, we no longer have city funding for our three composting sites or our compost outreach program. Big Reuse and eight other community composting organizations will not be able to continue our work without your continued support. Sign the petition to save community composting!
OUR PROGRAMS
Our Annual Impact
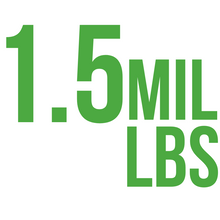
1.5 MIL lbs of used items diverted from the landfills.

2 MIL lbs of food scraps and yard waste processed into compost.

38K homes canvassed as we build NYC's city-wide composting program.
SHOP REUSE
Some of the latest vintage and collectable items available at the Reuse Center.













